Austin, TX, USA, Feb. 03, 2025 (GLOBE NEWSWIRE) — Custom Market Insights has published a new research report titled “Pharmacovigilance and Drug Safety Software Market Size, Trends and Insights By Functionality (Adverse Event Reporting Software, Drug Safety Audits Software, Issue Tracking Software, Fully Integrated Software), By Delivery Mode (On-premises, Cloud-based), By End User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Business Process Outsourcing Firms, Others), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033“ in its research database.
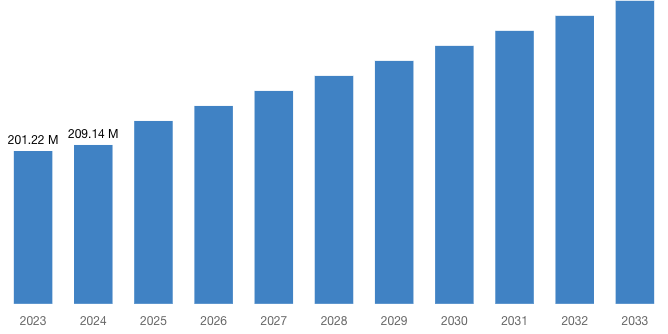
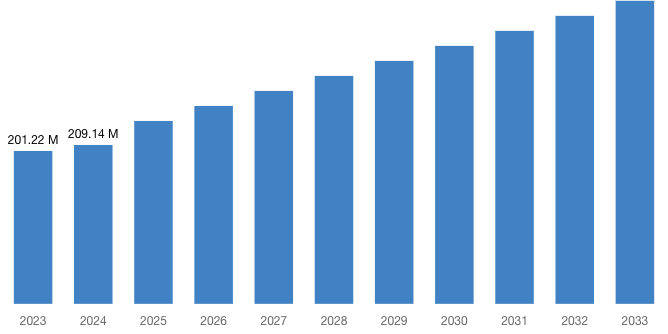
“According to the latest research study, the demand of global Pharmacovigilance and Drug Safety Software Market size & share was valued at approximately USD 201.22 Million in 2023 and is expected to reach USD 209.14 Million in 2024 and is expected to reach a value of around USD 398.96 Million by 2033, at a compound annual growth rate (CAGR) of about 6.8% during the forecast period 2024 to 2033.”
Click Here to Access a Free Sample Report of the Global Pharmacovigilance and Drug Safety Software Market @ https://www.custommarketinsights.com/request-for-free-sample/?reportid=51659
harmacovigilance and Drug Safety Software Market: Growth Factors and Dynamics
- Harnessing Advanced Data Processing Technologies: The Pharmacovigilance and Drug Safety Software market is witnessing substantial growth driven by advancements in data processing technologies. Technologies such as artificial intelligence, machine learning algorithms, and big data analytics are transforming how pharmaceutical companies monitor drug safety and comply with regulatory requirements. Pharmacovigilance solutions enable real-time monitoring of adverse drug reactions, ensuring patient safety and regulatory compliance across global markets.
- Technological Innovations in Pharmacovigilance: Innovations in cloud computing, natural language processing, and predictive analytics are reshaping the Pharmacovigilance landscape, offering scalable and agile safety monitoring solutions. Integration of these technologies enhances signal detection, risk assessment, and pharmacovigilance reporting capabilities. Continuous advancements in Pharmacovigilance software enable proactive risk management and expedited regulatory submissions, supporting pharmaceutical companies in delivering safe and effective medicines worldwide.
- Enhancing Patient Safety through Vigilant Monitoring: Pharmacovigilance and Drug Safety Software solutions play a crucial role in enhancing patient safety by monitoring drug interactions, adverse events, and medication errors in real time. These solutions facilitate early detection of safety signals, enabling timely interventions and mitigation strategies to minimize potential risks to patients’ health. From clinical trials to post-marketing surveillance, Pharmacovigilance systems uphold rigorous safety standards and regulatory compliance, ensuring public trust in pharmaceutical products.
- Collaboration and Regulatory Compliance: Strategic collaborations among pharmaceutical companies, regulatory agencies, and technology providers drive innovation and market growth in the Pharmacovigilance sector. Partnerships foster knowledge sharing, regulatory intelligence integration, and co-development of customized safety monitoring solutions. Collective efforts bolster pharmacovigilance capabilities, streamline adverse event reporting processes, and promote global harmonization of drug safety standards.
Request a Customized Copy of the Pharmacovigilance and Drug Safety Software Market Report @ https://www.custommarketinsights.com/inquire-for-discount/?reportid=51659
Pharmacovigilance and Drug Safety Software Market: COVID-19 Analysis
- Pandemic Impact and Resilient Healthcare Delivery: The COVID-19 pandemic underscored the critical role of Pharmacovigilance in ensuring patient safety amidst healthcare disruptions. Pharmaceutical companies relied on advanced drug safety software to monitor COVID-19 vaccine safety profiles, manage adverse reactions, and facilitate rapid regulatory approvals. Pharmacovigilance-driven safety monitoring systems supported global vaccination efforts and bolstered public confidence in pandemic response strategies.
- Accelerated Digital Transformation: The pandemic accelerated digital transformation initiatives in the Pharmacovigilance market, prompting investment in cloud-based safety monitoring platforms, real-time data analytics, and AI-driven safety signal detection algorithms. Agile Pharmacovigilance frameworks enabled rapid adaptation to evolving regulatory requirements and healthcare demands, reinforcing resilience in drug safety monitoring and pharmacovigilance operations.
- Future Growth Prospects and Recovery Strategies: Government investments in healthcare infrastructure, regulatory reforms, and pharmacovigilance capacity building initiatives stimulate market recovery and expansion. Pharmaceutical companies prioritize scalable Pharmacovigilance solutions, proactive risk management strategies, and AI-enhanced safety monitoring capabilities to navigate post-pandemic challenges. Emerging opportunities in personalized medicine, biopharmaceutical innovation, and global regulatory convergence are poised to drive sustained growth in the Pharmacovigilance sector.
Report Scope
| Feature of the Report | Details |
| Market Size in 2024 | USD 209.14 Million |
| Projected Market Size in 2033 | USD 398.96 Million |
| Market Size in 2023 | USD 201.22 Million |
| CAGR Growth Rate | 6.8% CAGR |
| Base Year | 2023 |
| Forecast Period | 2024-2033 |
| Key Segment | By Functionality, Delivery Mode, End User and Region |
| Report Coverage | Revenue Estimation and Forecast, Company Profile, Competitive Landscape, Growth Factors and Recent Trends |
| Regional Scope | North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America |
| Buying Options | Request tailored purchasing options to fulfil your requirements for research. |
(A free sample of the Pharmacovigilance and Drug Safety Software report is available upon request; please contact us for more information.)
Our Free Sample Report Consists of the following:
- Introduction, Overview, and in-depth industry analysis are all included in the 2024 updated report.
- The COVID-19 Pandemic Outbreak Impact Analysis is included in the package.
- About 220+ Pages Research Report (Including Recent Research)
- Provide detailed chapter-by-chapter guidance on the Request.
- Updated Regional Analysis with a Graphical Representation of Size, Share, and Trends for the Year 2024
- Includes Tables and figures have been updated.
- The most recent version of the report includes the Top Market Players, their Business Strategies, Sales Volume, and Revenue Analysis
- Custom Market Insights (CMI) research methodology
(Please note that the sample of the Pharmacovigilance and Drug Safety Software report has been modified to include the COVID-19 impact study prior to delivery.)
Request a Customized Copy of the Pharmacovigilance and Drug Safety Software Market Report @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
Pharmacovigilance and Drug Safety Software Market: Future Outlook and Opportunities
- Advancements in AI-driven Analytics and Safety Monitoring: The Pharmacovigilance market evolves with advancements in AI-driven analytics, real-world evidence integration, and predictive safety modeling capabilities. Integration of machine learning algorithms enhances adverse event detection, safety signal prioritization, and risk management efficacy in clinical research and post-marketing surveillance. Pharmacovigilance solutions become indispensable for ensuring medication safety, regulatory compliance, and patient-centric healthcare delivery.
- Market Expansion and Industry Adoption: The Pharmacovigilance market expands into new therapeutic areas and geographic regions, addressing diverse safety monitoring needs and compliance requirements. Increasing demand for integrated safety databases, pharmacovigilance automation tools, and patient outcome tracking systems drives market penetration and service innovation across global pharmaceutical ecosystems.
- Ethical Data Management and Regulatory Compliance: Continuous investment in ethical data management practices, transparent reporting frameworks, and regulatory compliance ensures patient confidentiality and regulatory adherence in Pharmacovigilance operations. The adoption of blockchain technology, secure cloud infrastructures, and data privacy regulations promotes sustainable pharmacovigilance practices and operational resilience in a data-driven healthcare environment.
- Pharmacovigilance as a Catalyst for Healthcare Innovation: As pharmaceutical companies embrace digital transformation powered by advanced safety monitoring technologies and collaborative partnerships, Pharmacovigilance solutions redefine patient safety, regulatory compliance, and therapeutic efficacy in global healthcare markets. The Pharmacovigilance sector emerges as a cornerstone of pharmaceutical risk management, supporting agile drug development processes, evidence-based medicine, and proactive healthcare decision-making.
The Pharmacovigilance and Drug Safety Software market is poised for significant growth driven by technological innovations, regulatory advancements, and strategic collaborations. As pharmaceutical companies leverage advanced Pharmacovigilance solutions to ensure drug safety and regulatory compliance, the market evolves to meet evolving healthcare demands, foster sustainable practices, and accelerate global healthcare innovation.
Request a Customized Copy of the Pharmacovigilance and Drug Safety Software Market Report @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
Key questions answered in this report:
- What is the size of the Pharmacovigilance and Drug Safety Software market and what is its expected growth rate?
- What are the primary driving factors that push the Pharmacovigilance and Drug Safety Software market forward?
- What are the Pharmacovigilance and Drug Safety Software Industry’s top companies?
- What are the different categories that the Pharmacovigilance and Drug Safety Software Market caters to?
- What will be the fastest-growing segment or region?
- In the value chain, what role do essential players play?
- What is the procedure for getting a free copy of the Pharmacovigilance and Drug Safety Software market sample report and company profiles?
Key Offerings:
- Market Share, Size & Forecast by Revenue | 2024−2033
- Market Dynamics – Growth Drivers, Restraints, Investment Opportunities, and Leading Trends
- Market Segmentation – A detailed analysis by Types of Services, by End-User Services, and by regions
- Competitive Landscape – Top Key Vendors and Other Prominent Vendors
Buy this Premium Pharmacovigilance and Drug Safety Software Research Report | Fast Delivery Available – [220+ Pages] @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
Pharmacovigilance and Drug Safety Software Market: Regional Analysis
- North America boasts a robust and well-established pharmaceutical industry, with numerous companies engaged in drug discovery, development, and commercialization. The region is a leader in technological innovation, particularly in healthcare and information technology. It benefits from a strong IT infrastructure, a favorable investment climate for research and development, and a significant emphasis on adopting digital solutions. North American pharmaceutical companies and research institutions frequently collaborate with academic institutions, healthcare providers, and technology companies to drive innovation in pharmacovigilance and drug safety. These collaborations promote the development and adoption of cutting-edge software solutions, further cementing North America’s dominance in the market. Additionally, the region’s stringent regulatory requirements for drug safety and pharmacovigilance are crucial factors driving the market’s growth in North America. Europe: Europe holds a substantial market share driven by advanced healthcare infrastructure and stringent drug safety regulations.
- Europe holds a significant position in the pharmacovigilance and drug safety software market, supported by a well-established pharmaceutical industry, rigorous regulatory standards, and a growing focus on real-world data and patient safety. Leading countries such as the United Kingdom, Germany, and France contribute prominently to the European market. They are driving initiatives to integrate digital health technologies, enhance pharmacovigilance capabilities, and ensure compliance with EU regulations governing drug safety. Key trends influencing the European market include the increasing adoption of cloud-based pharmacovigilance solutions, advancements in AI and predictive analytics, and collaborative efforts among European pharmacovigilance networks. These developments are shaping the landscape and driving innovation in pharmacovigilance and drug safety across Europe.
- Asia Pacific is experiencing rapid expansion in the pharmacovigilance and drug safety software market, propelled by a burgeoning pharmaceutical sector, increased healthcare expenditure, and heightened awareness of medication safety. Key markets in the region include China, India, and Japan, where governments are actively promoting initiatives to bolster pharmacovigilance infrastructure, improve regulatory adherence, and address concerns related to medication safety. The market growth in Asia Pacific is further driven by the growing adoption of pharmacovigilance software among pharmaceutical firms, contract research organizations (CROs), and healthcare providers. Additionally, advancements in digital health technologies are playing a pivotal role in accelerating the adoption and development of pharmacovigilance solutions across the region. The pharmacovigilance and drug safety software market is poised for significant growth, driven by the increasing need for efficient adverse event monitoring, regulatory compliance, and technological advancements. Key players are focusing on enhancing their software capabilities through innovations in AI, machine learning, and cloud computing to meet the evolving needs of the pharmaceutical industry. Addressing challenges such as data privacy concerns, integration issues, and high implementation costs will be crucial for maximizing the potential of pharmacovigilance software in ensuring drug safety and regulatory adherence globally.
Request a Customized Copy of the Pharmacovigilance and Drug Safety Software Market Report @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
(We customized your report to meet your specific research requirements. Inquire with our sales team about customizing your report.)
Still, Looking for More Information? Do OR Want Data for Inclusion in magazines, case studies, research papers, or Media?
Email Directly Here with Detail Information: support@custommarketinsights.com
Browse the full “Pharmacovigilance and Drug Safety Software Market Size, Trends and Insights By Functionality (Adverse Event Reporting Software, Drug Safety Audits Software, Issue Tracking Software, Fully Integrated Software), By Delivery Mode (On-premises, Cloud-based), By End User (Pharmaceutical and Biotechnology Companies, Contract Research Organizations, Business Process Outsourcing Firms, Others), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033“ Report at https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
List of the prominent players in the Pharmacovigilance and Drug Safety Software Market:
- Oracle Corporation (US)
- ArisGlobal LLC (US)
- Ennov (France)
- AB Cube (France)
- United BioSource LLC (US)
- Sparta Systems Inc. (US)
- Veeva Systems (US)
- Online Business Applications Inc. (US)
- Sarjen Systems Pvt. Ltd. (India)
- Exponential HealthTech (India)
- LORENZ Life Sciences Group (Germany)
- PAREXEL International Corporation (US)
- Cognizant Technology Solutions (US)
- Capgemini SE (France)
- Accenture (Ireland)
- Others
Click Here to Access a Free Sample Report of the Global Pharmacovigilance and Drug Safety Software Market @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
Spectacular Deals
- Comprehensive coverage
- Maximum number of market tables and figures
- The subscription-based option is offered.
- Best price guarantee
- Free 35% or 60 hours of customization.
- Free post-sale service assistance.
- 25% discount on your next purchase.
- Service guarantees are available.
- Personalized market brief by author.
Browse More Related Reports:
Empty Capsules Market: Empty Capsules Market Size, Trends and Insights By Type (Gelatin Capsules, Non-gelatin Capsules), By Functionality (Immediate-release Capsules, Sustained-release Capsules, Delayed-release Capsules), By Application (Antibiotic & Antibacterial Drugs, Vitamin & Dietary Supplements, Antacid & Antiflatulent Preparations, Cardiovascular Therapy Drugs, Other), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Digital Holography Microscopy Market: Digital Holography Microscopy Market Size, Trends and Insights By Offering (Hardware, Software), By End User (Рhаrmасеutісаl & Віоtесhnоlоgу Соmраnіеѕ, Rеѕеаrсh Оrgаnіzаtіоnѕ, Асаdеmіс Меdісаl Сеntеrѕ, Ноѕріtаlѕ & Сlіnісѕ, Others), By Type (Lаbеl-Frее Іntеrfеrоmеtrіс Тесhnіquе, Quаntіtаtіvе Рhаѕе Іmаgе), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2025–2034
Surgical Site Infection Control Market: Surgical Site Infection Control Market Size, Trends and Insights By Product (Surgical scrubs, Hair clippers, Surgical drapes, Surgical irrigation), By Surgery/Procedure (Cataract surgery, Cesarean section, Dental restoration, Gastric bypass, Others), By Type of Infection (Superficial incisional SSI, Deep incisional SSI, Organ or space SSI), By End-use (Hospitals, Ambulatory surgical centers, Others), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033
Clinical Trial Outsourcing Market: Clinical Trial Outsourcing Market Size, Trends and Insights By Services (Protocol Designing, Site Identification, Patient Recruitment, Laboratory Services, Bioanalytical Testing Services, Clinical Trial Data Management Services, Others), By Phase (Phase I, Phase II, Phase III, Phase IV), By Study Design (Interventional, Observational, Expanded Access), By Applications (Cancer, Cardiovascular Diseases, Nervous System Diseases, Infectious Diseases, Musculoskeletal Disease, Gastroenterology Diseases, Others), By End-User (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, Others), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033
Veterinary Orthopedic Implants Market: Veterinary Orthopedic Implants Market Size, Trends and Insights By Product Type (Plates, Screws, Others), By Application (Cruciate Ligament Rupture, Bone Fractures, Elbow Dysplasia, Hip Dysplasia, Others), By End User (Veterinary Hospitals, Veterinary Clinics, Others), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033
Drug Addiction Treatment Market: Global Drug Addiction Treatment Market Size, Trends and Insights By Type (Opioid Addiction, Benzodiazepine Addiction, Barbiturate Addiction, Others), By Treatment (Therapy, Medication, Others), By Route of Administration (Oral, Parenteral, Others), By End Users (Hospitals, Specialty Clinics, Others), By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacies, Others), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033
US Veterinary Equipment and Disposables Market: US Veterinary Equipment and Disposables Market Size, Trends and Insights By Product (Disposables/ Consumables, Equipment & Accessories), By Animal Type (Large Animals, Small Animals), By Usage (Monitoring, Diagnostic, Surgical), By End-Use (Laboratories, Veterinary Clinics, Veterinary Hospitals, Others), and By Region – Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033
Animal Genetics Market: Animal Genetics Market Size, Trends and Insights By Animal Type (Poultry, Porcine, Bovine, Canine, Feline, Others), By Genetic Method (Semen, Embryos, Live Animals, Genetic Testing), By Service Type (Genetic Trait Testing, Genetic Disease Testing, Animal Genetic Products, Others), By Application (Dairy Production, Meat Production, Poultry Production, Aquaculture, Companion Animals, Others), By End User (Veterinary Hospitals & Clinics, Animal Breeding Centers, Research Centers & Institutes, Diagnostic Centers), and By Region – Global Industry Overview, Statistical Data, Competitive Analysis, Share, Outlook, and Forecast 2024–2033
The Pharmacovigilance and Drug Safety Software Market is segmented as follows:
By Functionality
- Adverse Event Reporting Software
- Drug Safety Audits Software
- Issue Tracking Software
- Fully Integrated Software
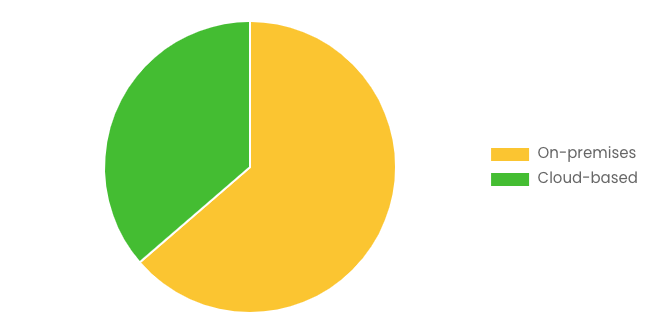
By Delivery Mode
By End User
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
- Business Process Outsourcing Firms
- Others
Click Here to Get a Free Sample Report of the Global Pharmacovigilance and Drug Safety Software Market @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
Regional Coverage:
North America
- U.S.
- Canada
- Mexico
- Rest of North America
Europe
- Germany
- France
- U.K.
- Russia
- Italy
- Spain
- Netherlands
- Rest of Europe
Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Taiwan
- Rest of Asia Pacific
The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
Latin America
- Brazil
- Argentina
- Rest of Latin America
This Pharmacovigilance and Drug Safety Software Market Research/Analysis Report Contains Answers to the following Questions.
- Which Trends Are Causing These Developments?
- Who Are the Global Key Players in This Pharmacovigilance and Drug Safety Software Market? What are Their Company Profile, Product Information, and Contact Information?
- What Was the Global Market Status of the Pharmacovigilance and Drug Safety Software Market? What Was the Capacity, Production Value, Cost and PROFIT of the Pharmacovigilance and Drug Safety Software Market?
- What Is the Current Market Status of the Pharmacovigilance and Drug Safety Software Industry? What’s Market Competition in This Industry, Both Company and Country Wise? What’s Market Analysis of Pharmacovigilance and Drug Safety Software Market by Considering Applications and Types?
- What Are Projections of the Global Pharmacovigilance and Drug Safety Software Industry Considering Capacity, Production and Production Value? What Will Be the Estimation of Cost and Profit? What Will Be Market Share, Supply and Consumption? What about imports and exports?
- What Is Pharmacovigilance and Drug Safety Software Market Chain Analysis by Upstream Raw Materials and Downstream Industry?
- What Is the Economic Impact On Pharmacovigilance and Drug Safety Software Industry? What are Global Macroeconomic Environment Analysis Results? What Are Global Macroeconomic Environment Development Trends?
- What Are Market Dynamics of Pharmacovigilance and Drug Safety Software Market? What Are Challenges and Opportunities?
- What Should Be Entry Strategies, Countermeasures to Economic Impact, and Marketing Channels for Pharmacovigilance and Drug Safety Software Industry?
Click Here to Access a Free Sample Report of the Global Pharmacovigilance and Drug Safety Software Market @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
Reasons to Purchase Pharmacovigilance and Drug Safety Software Market Report
- Pharmacovigilance and Drug Safety Software Market Report provides qualitative and quantitative analysis of the market based on segmentation involving economic and non-economic factors.
- Pharmacovigilance and Drug Safety Software Market report outlines market value (USD) data for each segment and sub-segment.
- This report indicates the region and segment expected to witness the fastest growth and dominate the market.
- Pharmacovigilance and Drug Safety Software Market Analysis by geography highlights the consumption of the product/service in the region and indicates the factors affecting the market within each region.
- The competitive landscape incorporates the market ranking of the major players, along with new service/product launches, partnerships, business expansions, and acquisitions in the past five years of companies profiled.
- Extensive company profiles comprising company overview, company insights, product benchmarking, and SWOT analysis for the major market players.
- The Industry’s current and future market outlook concerning recent developments (which involve growth opportunities and drivers as well as challenges and restraints of both emerging and developed regions.
- Pharmacovigilance and Drug Safety Software Market Includes in-depth market analysis from various perspectives through Porter’s five forces analysis and provides insight into the market through Value Chain.
Reasons for the Research Report
- The study provides a thorough overview of the global Pharmacovigilance and Drug Safety Software market. Compare your performance to that of the market as a whole.
- Aim to maintain competitiveness while innovations from established key players fuel market growth.
Buy this Premium Pharmacovigilance and Drug Safety Software Research Report | Fast Delivery Available – [220+ Pages] @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
What does the report include?
- Drivers, restrictions, and opportunities are among the qualitative elements covered in the worldwide Pharmacovigilance and Drug Safety Software market analysis.
- The competitive environment of current and potential participants in the Pharmacovigilance and Drug Safety Software market is covered in the report, as well as those companies’ strategic product development ambitions.
- According to the component, application, and industry vertical, this study analyzes the market qualitatively and quantitatively. Additionally, the report offers comparable data for the important regions.
- For each segment mentioned above, actual market sizes and forecasts have been given.
Who should buy this report?
- Participants and stakeholders worldwide Pharmacovigilance and Drug Safety Software market should find this report useful. The research will be useful to all market participants in the Pharmacovigilance and Drug Safety Software industry.
- Managers in the Pharmacovigilance and Drug Safety Software sector are interested in publishing up-to-date and projected data about the worldwide Pharmacovigilance and Drug Safety Software market.
- Governmental agencies, regulatory bodies, decision-makers, and organizations want to invest in Pharmacovigilance and Drug Safety Software products’ market trends.
- Market insights are sought for by analysts, researchers, educators, strategy managers, and government organizations to develop plans.
Request a Customized Copy of the Pharmacovigilance and Drug Safety Software Market Report @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/
About Custom Market Insights:
Custom Market Insights is a market research and advisory company delivering business insights and market research reports to large, small, and medium-scale enterprises. We assist clients with strategies and business policies and regularly work towards achieving sustainable growth in their respective domains.
CMI provides a one-stop solution for data collection to investment advice. The expert analysis of our company digs out essential factors that help to understand the significance and impact of market dynamics. The professional experts apply clients inside on the aspects such as strategies for future estimation fall, forecasting or opportunity to grow, and consumer survey.
Follow Us: LinkedIn | Twitter | Facebook | YouTube
Contact Us:
Joel John
CMI Consulting LLC
1333, 701 Tillery Street Unit 12,
Austin, TX, Travis, US, 78702
USA: +1 801-639-9061
India: +91 20 46022736
Email: support@custommarketinsights.com
Web: https://www.custommarketinsights.com/
Blog: https://www.techyounme.com/
Blog: https://atozresearch.com/
Blog: https://www.technowalla.com/
Blog: https://marketresearchtrade.com/
Buy this Premium Pharmacovigilance and Drug Safety Software Research Report | Fast Delivery Available – [220+ Pages] @ https://www.custommarketinsights.com/report/pharmacovigilance-and-drug-safety-software-market/

© 2025 Benzinga.com. Benzinga does not provide investment advice. All rights reserved.